Symbols Glossary
United States Title 21 of the Code of Federal Regulations (CFR) Part 801 - Labeling
| Symbol | Symbol Title | Explanatory Text | Standard Reference |
|---|---|---|---|
| Prescription Use Only | CAUTION: Federal (USA) law restricts this device to sale by or on the order of a licensed healthcare practitioner | Section 801.109 (b) (1) |
ISO-15223-1 Medical devices – Symbols to be used with medical device labels, labelling and information to be supplied – Part 1: General requirements
| Symbol | Symbol Title | Explanatory Text | Standard Reference |
|---|---|---|---|
| Manufacturer | Indicates the medical device manufacturer. | 5.1.1 | |
| Authorized Representative in the European Community | Indicates the authorized representative in the European Community / European Union. | 5.1.2 | |
| Date of Manufacture | Indicates the date when the medical device was manufactured. | 5.1.3 | |
| Use-by date | Indicates the date after which the medical device is not be used. | 5.1.4 | |
| Batch code | Indicates the manufacturer’s batch code so that the batch or lot can be identified. | 5.1.5 | |
| Catalogue number | Indicates the manufacturer’s catalogue number so that the medical device can be identified. | 5.1.6 | |
 | Importer | Indicates the entity importing the medical device into the locale. | 5.1.8 |
| Sterile | Indicates a medical device that has been subjected to a sterilization process. | 5.2.1 | |
| Sterilized by ethylene oxide treatment | Indicates a medical device that has been sterilized using ethylene oxide. | 5.2.3 | |
| Sterilized using irradiation | Indicates a medical device that has been sterilized using irradiation. | 5.2.4 | |
| Do Not Re-sterilize | Indicates a medical device that is not to be re-sterilized. | 5.2.6 | |
| Non-Sterile | Indicates a medical device that has not been subjected to a sterilization process | 5.2.7 | |
| Do not use if package is damaged and consult instructions for use | Indicates a medical device that should not be used if the package has been damaged or opened and that the user should consult the instructions for use for additional information. | 5.2.8 | |
| Single Sterile Barrier System | Indicates a Single Sterile Barrier System | 5.2.11 | |
| Double Sterile Barrier System | Indicates a Double Sterile Barrier System | 5.2.12 | |
| Keep away from sunlight | Indicates a medical device that needs protection from light sources. | 5.3.2 | |
| Keep Dry | Indicates a medical device needs to be protected from moisture. | 5.3.4 | |
| Upper limit of temperature | Indicates the upper limit of temperature to which the medical device can be safely exposed. | 5.3.6 | |
| Temperature limit | Indicates the temperature limits to which the medical device can be safely exposed. | 5.3.7 | |
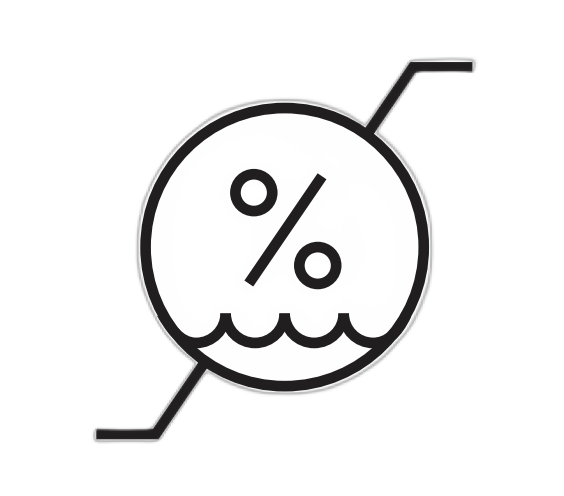 | Humidity Limitation | Indicates the range of humidity to which the medical device can be safely exposed. | 5.3.8 |
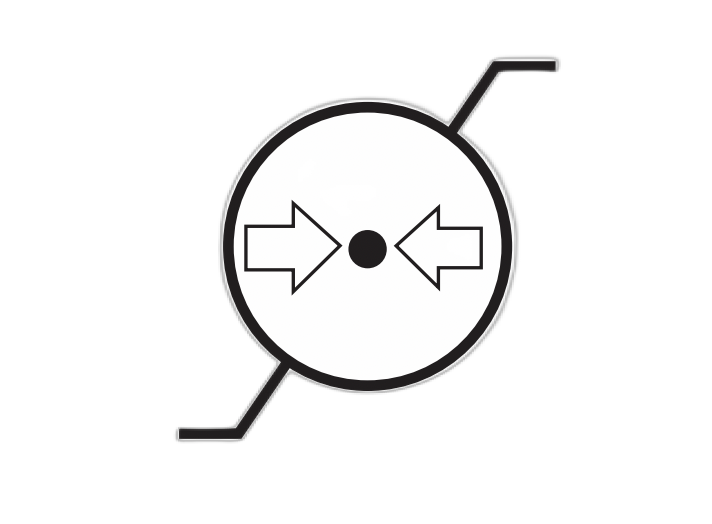 | Atmospheric Pressure Limitation | Indicates the range of atmospheric pressure to which the medical device can be safely exposed. | 5.3.9 |
| Do not re-use | Indicates a medical device that is intended for one single use only. | 5.4.2 | |
| Consult instructions for use or consult electronic instructions for use. | Indicates the need for the user to consult the instructions for use. | 5.4.3 | |
| Caution | Indicates that caution is necessary when operating the device or control close to where the symbol is placed, or that the current situation needs operator awareness or operator action in order to avoid undesirable consequences. | 5.4.4 | |
 | Contains latex or presence of natural rubber | Indicates the presence of natural rubber or dry natural rubber latex as a material of construction within the medical device or the packaging of a medical device. | 5.4.5 |
| Not made with natural rubber latex | Indicates that neither the medical device nor the packaging of the medical device contains the presence of natural rubber latex. | 5.4.5 and Annex B.2 and Guidance for Industry and US Food and Drug Administration Staff - Recommendations for Labeling Medical Products to Inform Users that the Product or Product Container is not Made with Natural Rubber Latex; Issued on December 2, 2014 |
|
| Medical Device | Indicates the item is a Medical Device. | 5.7.7 |
European Medical Device Directive 93/42/EEC / European Medical Device Regulation 2017/745
| Symbol | Symbol Title | Explanatory Text | Standard Reference |
|---|---|---|---|
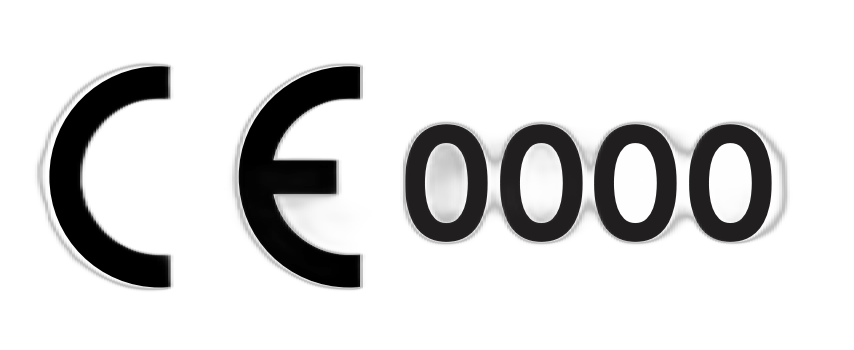 | Article 17 / Article 20 | CE Conformity Marking and Notified Body Number | Product conforms to the applicable requirements for a medical device as set forth in the regulation and assessed by the certifying notified body. |
| Article 17 / Article 20 | CE Conformity Marking | Product conforms to the applicable requirements for medical device as set forth in the regulation. |
BS EN 15986:2011 – Medical devices – Symbol for use in the labelling of medical devices. Requirements for labelling of medical devices containing phthalates
| Symbol | Symbol Title | Explanatory Text | Standard Reference |
|---|---|---|---|
| Does not contain DEHP | Indicates a medical device that does not contain the phthalate plasticizers DEHP | Annex B | |
| Contains or presence of phthalate DEHP | Indicates presence of Bis (2-ethylexyl) phthalate (DEHP). | A.2 |
IEC 60601-1 Medical electrical equipment - Part 1: General requirements for basic safety and essential performance
| Symbol | Symbol Title | Explanatory Text | Standard Reference |
|---|---|---|---|
| Degree of Ingress Protection Provided by Enclosure | Degree of protection against the penetration of solid foreign matter and liquids. Protected against solid objects greater than or equal to 12.5 mm diameter and protected against the effects of temporary immersion in water. | Table D.3, Symbol 2 IEC 60529 |
|
 | Type BF applied part | To identify a type BF applied part complying with IEC 60601-1. | Table D.1, Symbol 20 |
Other Symbols
| Symbol | Symbol Title | Explanatory Text | Standard Reference |
|---|---|---|---|
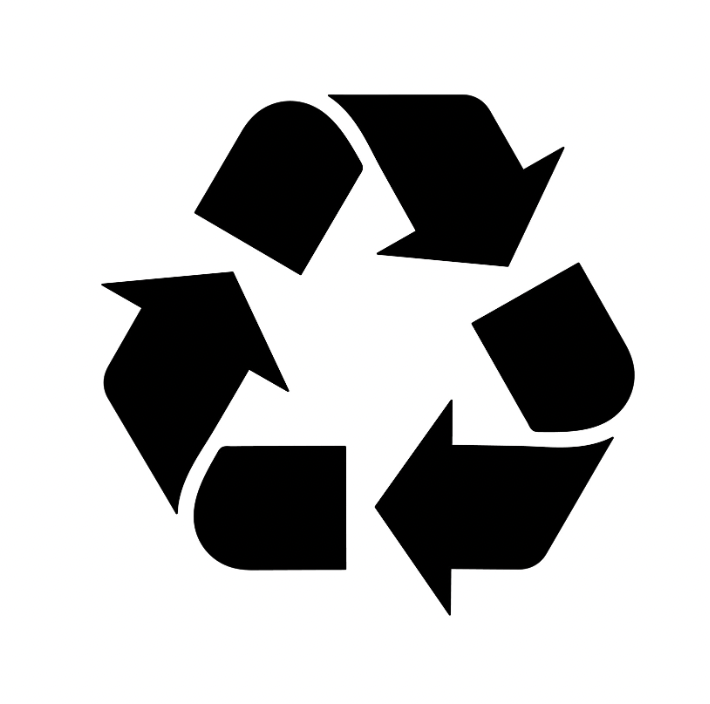 | Recyclable | Product packaging is recyclable. | N/A |
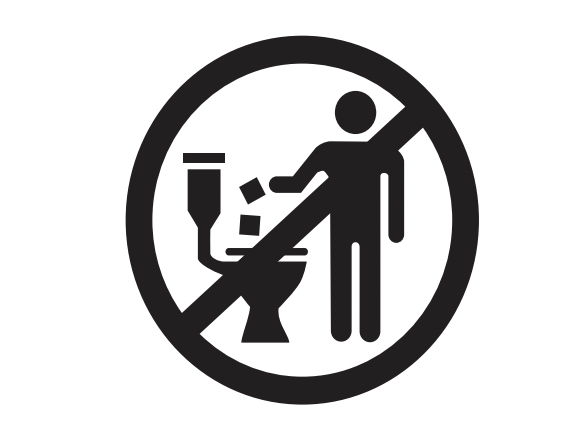 | Do not flush | Likely to be used in a bathroom with significant potential to be flushed. | N/A |
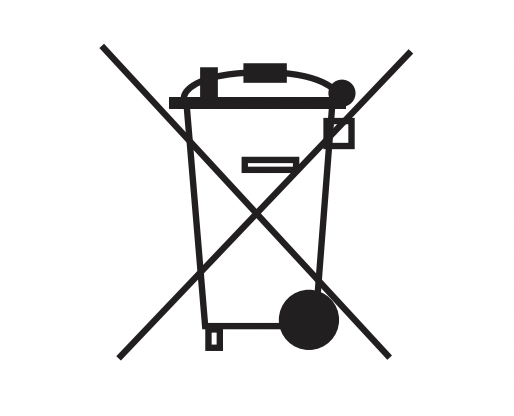 | Recycle: Electronic Equipment | Do not dispose of this product in unsorted municipal waste stream. | EN 50419 |
 | MR Conditional | Item with demonstrated safety in the MR environment within defined conditions including conditions for the static magnetic field, the time-varying gradient magnetic fields, and the radiofrequency fields. | ASTM F2503 Table 2 |
Therapeutic Goods Medical Device Regulation
| Symbol | Symbol Title | Explanatory Text | Standard Reference |
|---|---|---|---|
| Australian Sponsor | Indicates the authorized sponsor in the Australian market. | N/A |


